Chemistry is our fifth and final factor of clean in our series. Because of the complexity of this area, we will break the chemistry factor into two parts. The first part will focus on alkaline and acidic cleaners while part two will focus on surfactants and road film and will appear in the August issue of Professional Carwashing & Detailing.
Cleaning Chemistry is the reaction of the detergent solution with the soil to assist with its removal. We will look at the ingredients involved in these reactions.
There are two primary detergent building blocks in determining the chemistry of a detergent: the “Builder”portion which determines if the product is an alkaline, neutral or acidic; and the surfactant/solvent portion which provides other functionalities (we will cover in part 2).
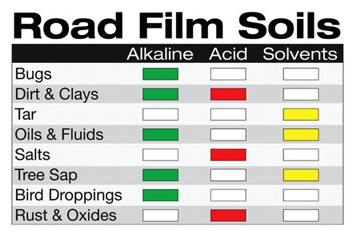
Alkaline detergents are the best overall cleaners, they are effective on organic soils such as oils, bugs, asphalt and tree sap. Acidic detergents are generally poor solo cleaners. They are almost always used in a two-step Alkaline/Acidic application but when used can effectively clean inorganic soils such as clay, sand and dirt. Specialized “Solvent”detergents are often used to remove very tough grease tar and tree sap as shown in the “Road Film Soils”table.
The key to achieving a clean car is to use the right product or products at the proper concentrations for the current cleaning conditions. For most Alkaline or Acidic detergents you can measure the concentrations using a titration kit.
The pH Scale
The pH scale indicates whether a product is alkaline or acidic. The pH reading is a measure of the concentration of Hydrogen Ions in the solution. It is reported as the negative logarithm of that concentration which means that the lower the number the greater the amount of Hydrogen Ions in the solution and the more acidic the solution. The pH range is from 0 which is acidic, to 14 which is alkaline, or Base with 7 being neutral.
Extremes on either end of the spectrum can be dangerous. Low pH or acidic products are more corrosive in general than alkaline. However, this can be deceiving if testing the pH when acidic fluorides such as HF, ABF and AF are involved. These are compounds that can produce solutions that may not be low in pH but are more dangerous due to their toxicity than their corrosivity.
pH vs. Alkalinity
Some chemical builders can easily drive the pH to near the end of the pH scale, but they may just as easily be neutralized. Other chemical builders may not have as dramatic effect on the pH but will help “buffer”or “sustain”the pH at some optimal range for that material. If you were to try to determine the dilution ratio of a detergent by using the pH of the solution, you may find that the pH does not change much with dilution if the solution is being buffered.
Different detergent builders contribute different amounts of alkalinity to the solution. The various concentrations of these builders can hold the pH of the detergent solution at each builder’s maximum pH potential as the solution is diluted.
Or for another way to look at this issue: suppose that you were offered a million dollars but you had to swim across a pool with piranhas to collect the money. If there was just one piranha in the pool, you might consider going for it. However, if there were 1,000 piranhas in the pool, you probably would not. With pH, it’s like saying “there are piranhas in the pool”but not how many. Alkalinity tells you how many.
When you use a titration kit you are neutralizing all of the buffering ability and comparing the number of drops of titrant used to a chart that indicates the number of drops needed to neutralize the product at various dilution ratios that was determined through testing.
Alkaline detergent builders
Alkalinity can assist with cleaning while creating a pH solution that is above 7 with high alkaline products making solutions that are generally above 11 in pH. Alkaline detergents are the best overall cleaners and can remove most soils. These alkaline detergents can be used as a solo detergent, using one or two applications, or in a two-step Alkaline/Acidic process. Remember that all cleaners have a point of diminishing returns and over application not only wastes money but may also cause damage.
Alkaline ingredients include Silicates, Hydroxides, Carbonates and Phosphates.
Silicates can add detergency, alkalinity and some protection against corrosive caustics in high alkaline solutions. There are a number of forms of silicates used in detergents, both powdered and liquid.
Caustics are commonly used in frictionless presoaks to react with the soils, making them more soluble and easier to remove. Sodium Hydroxide and Potassium Hydroxide are the most common caustics used in detergents. They can raise the pH very high and be quite corrosive. If used too strong and hot, caustics can also react with the surface of the vehicle so caution must be used when working with caustic products.
Phosphates assist with the dispersion, or removal, of soils and controlling small amounts of water hardness. Phosphates will buffer solutions in the mild alkaline range and are generally not very aggressive. Phosphates act as fertilizers if they get into surface waters and are therefore restricted in some states and areas.
Acidic builders
Acidic cleaners contain ingredients that assist with cleaning while keeping the pH of the solution less than pH 7. Acidic ingredients include mineral acids and organic acids.
Mineral acids such as Sulfuric and Phosphoric are generally strong acids that help remove mineral soils by attacking the mineral itself, but are not effective on oily soils. When Phosphoric Acid is neutralized it forms Phosphates so it may not be used in areas with Phosphate Bans.
Organic acids like citric acid and glycolic acid are generally less corrosive than mineral acids but often less effective on the mineral soils. There are, however, a wide variety of organic acids and the right ones in a well designed formulation can be quite effective. Again, they are not as effective as an alkaline detergent on oily soils.
Hydrofluoric acid (HF) and its salts ammonium bifluoride (ABF) and ammonium fluoride (AF) are very effective mineral acids but are too dangerous for any use in a car wash environment. These acids are very toxic and will damage vehicles, car wash equipment, and are extremely dangerous for humans, causing injury and death.
For the best cleaning results, it is important to be aware of current cleaning conditions and choose detergent products that are most effective for those conditions. It is also important to adjust those products for optimal performance along with considerations of the other four factors; water quality, mechanical action, temperature and time to achieve the best results.